Abstract
Background: CAR T-cell therapy is a novel treatment for patients with hematologic malignancies that carries risk of unique toxicities such as cytokine release syndrome (CRS) and immune effector cell-associated neurologic toxicity (ICANS). Patients are at risk for quality of life (QOL) deterioration and immense physical and psychological symptom burden given these toxicities, their illness, and the need for a prolonged hospitalization. Yet, data that comprehensively depict the longitudinal patient-reported outcomes (PROs) of patients receiving CAR T-cell therapy are limited.
Methods: We conducted a longitudinal cohort study of 100 consecutive adult patients with hematologic malignancies receiving CAR T-cell therapy at Massachusetts General Hospital (MGH) between 4/2019 and 11/2021. Patients completed baseline questionnaires prior to receipt of CAR T-cell therapy about sociodemographic data, QOL (Functional Assessment of Cancer Therapy-General), physical symptoms (Edmonton Symptom Assessment Survey-revised [ESAS-r]), and psychological distress (Hospital Anxiety and Depression Scale and Post-Traumatic Stress Checklist) and completed PRO questionnaires at 1 week, 1 month, 3 months, and 6 months after CAR T-cell infusion. We used descriptive statistics to summarize patient characteristics and PROs at each timepoint and rates of clinically significant depression, anxiety, and post-traumatic stress disorder (PTSD) based on establish cutoffs. We conducted paired t-tests analyzing changes in QOL, physical, and psychological symptoms from baseline to 1 week, 3 months, and 6 months. We used multivariable linear regression models to examine factors associated with QOL at three months after CAR T-cell infusion.
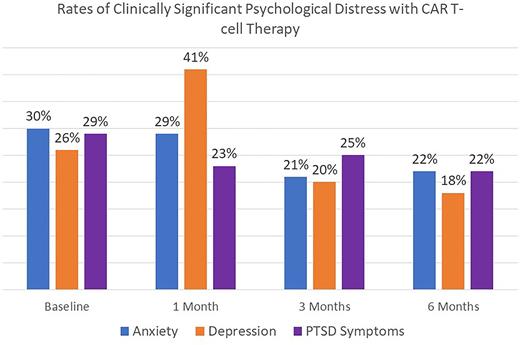
Results: We enrolled 71.4% (100/140) of eligible patients (median age 66 years; 63% male sex; 77% married/living with partner). The most common diagnosis was lymphoma (71%) followed by multiple myeloma (28%); A plurality (34%) received tisagenlecleucel, followed by lisocabtagene maraleucel (16%), axicabtagene ciloleucel (13%), and idecabtagene vicleucel (12%). The overall response rate was 80%, and with a median follow up of 14 months after CAR T-cell infusion, 38% died. CRS and ICANS occurred in 76% and 33% of patients, respectively. At baseline, median QOL score was 77.9 (range: 34-107), and was 70.0 (35-107) at 1 week, 76.0 (46-106) at 1 month, 83.5 (29-108) at 3 months, and 83.7 (34-107) at 6 months. Severe physical symptoms (ESAS-r score >=7) were reported by 47%, 52%, 35%, 28%, and 28% of patients at baseline, 1 week, 1 month, 3 months, and 6 months, respectively. The rates of clinically significant anxiety, depression, and PTSD symptoms at each time point are depicted in figure 1. Patient's QOL decreased at 1 week (mean difference [md]=5.1, p=0.04), but increased at 3 months (md -5.2, p=0.04) and 6 months (md=-5.3, p=0.07) compared to baseline. Patient's physical symptoms increased at 1 week, but decreased at 3 months (md 5.1, p=0.03) and 6 months (md=4.5, p=0.07), compared to baseline. There was no significant difference in anxiety symptoms from baseline to 1 week, 1 month, or 3 months. Anxiety symptoms improved at 6 months compared to baseline. There was no significant difference in depression or PTSD symptoms across all time points compared to baseline. In univariate linear regression, older age (β=0.4; p=0.01) and undergraduate or graduate degree (β=7.53, p=0.04) were associated with better QOL, whereas baseline anxiety symptoms (β=-14.3, p<0.01), baseline depression symptoms (β=-16.6, p<0.01), and baseline PTSD symptoms (β=-18.8, p<0.01) were associated with worse QOL at 3 months. In separate multivariable models including age, sex, education, marital status, and Eastern Cooperative Oncology Group performance status given collinearity among baseline PROs, anxiety (β=-10.6, p<0.01), depression (β=-12.6, p<0.01), and PTSD symptoms (β=-16.2, p<0.01) were the only factors associated with QOL at 3 months.
Conclusion: Patients receiving CAR T-cell therapy experience significant QOL impairment and physical and psychological symptom burden, which peaks 1 week after cell infusion and improves by 6 months post-infusion. Given high rates of persistent severe symptoms and clinically significant psychological distress, interventions to improve the QOL of these patients are warranted.
Disclosures
Johnson:AstraZeneca: Consultancy; ADC Therapeutics: Consultancy; Seagen: Consultancy. Frigault:Kite/Gilead: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Arcellx: Research Funding; JnJ/Legend: Consultancy; Iovance: Consultancy; Cytoagents: Consultancy; BMS: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal